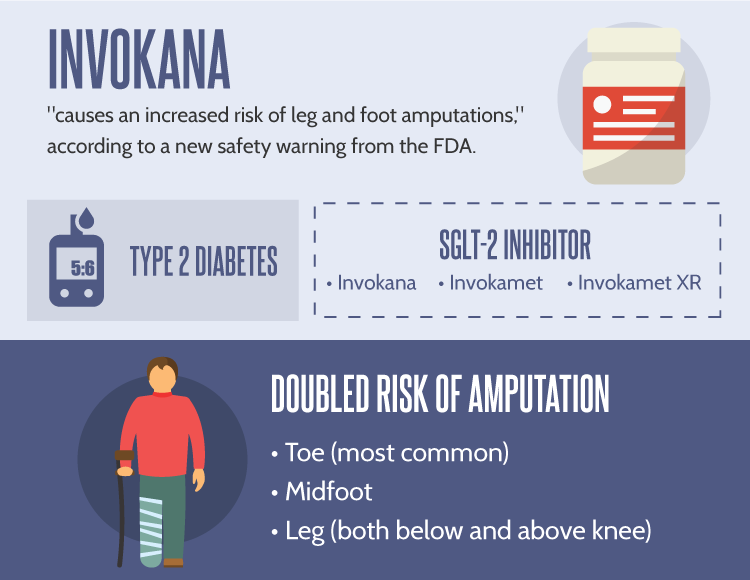
The new type 2 diabetes drug Invokana has been linked to a host of severe side effects, but now the US Food & Drug Administration has warned patients about a risk for foot and leg amputations:
- Toe amputation (most common)
- Doubled risk over placebo
Contact the experienced lawyers at Monheit Law today for a free legal consultation.
We’re prepared to fight for type 2 diabetes patients.
Troubling results from two recently-completed studies suggest that canagliflozin, the active ingredient in Invokana, causes an increase in the risk for leg, foot and toe amputations. A new black box warning has been added to product labeling for Invokana, Invokamet and Invokamet XR.
Feel free to share our Invokana infographic on your own site. Just copy and paste the code below. Please include attribution to MonheitLaw.com.
FREE CONSULTATION
Current Invokana patients should watch for pain and tenderness in the feet, legs or toes, while physicians are instructed to screen prospective patients for factors that increase the risk of amputation.
Invokana Amputation Lawsuits
Hundreds of type 2 diabetes patients have already filed Invokana lawsuits against Janssen Pharmaceutical, the drug’s manufacturer and a subsidiary of global giant Johnson & Johnson. Today, more than 850 lawsuits are consolidated in the US District Court of New Jersey, claiming Invokana causes multiple severe complications, including diabetic ketoacidosis and kidney failure. With new medical evidence confirming a link between Invokana and amputations, legal observers believe that hundreds of other type 2 diabetes patients may also be able to file suit.
Increased Risk For Foot & Leg Amputations
New statistical analyses confirm that patients taking Invokana, Invokamet or Invokamet XR are around twice as likely to require leg and foot amputations than patients taking a placebo.
Alarmed by these recent findings, experts at the US Food & Drug Administration have now formally warned patients of Invokana’s newly-identified risk. On May 16, 2017, the FDA issued an urgent safety announcement, citing new data from two major clinical trials that investigated the drug’s potential dangers for eight years.
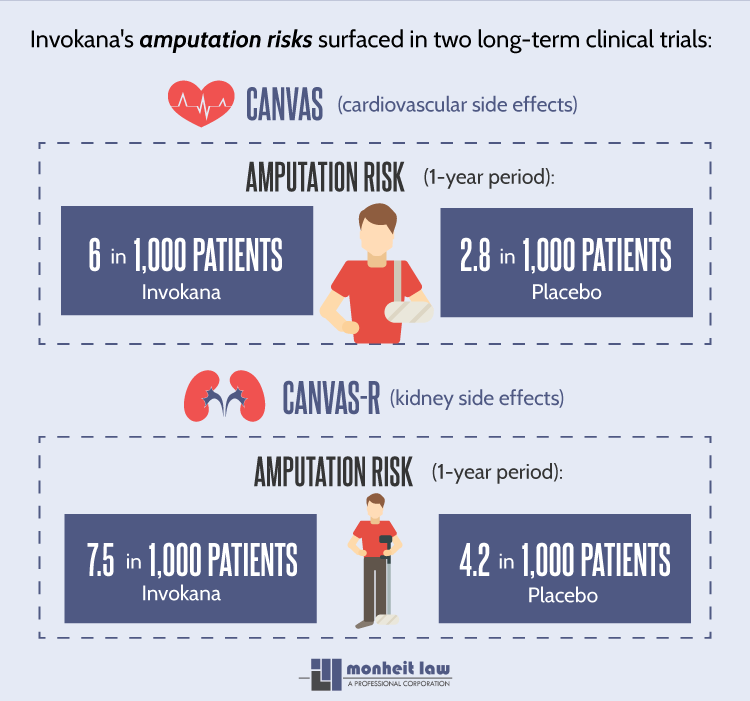
CANVAS & CANVAS-R
The risk for limb amputations, however, only became conclusive once these trials, known in medical circles as CANVAS and CANVAS-R, were completed in July 2017.
Want to share this infographic on your site? Use the code below, but please include attribution to MonheitLaw.com:
In the CANVAS trial, designed to monitor the drug’s effect on cardiovascular health, nearly 6 in every 1,000 patients on canagliflozin underwent a leg and / or foot amputation. Leg and foot amputations occurred in only 2.8 in 1,000 patients taking the placebo. A similar trend was observed in CANVAS-R, established to gauge Invokana’s potential kidney risks.
While 4.2 out of 1,000 placebo patients underwent amputations, 7.5 in 1,000 canagliflozin patients met the same fate. These risks are calculated on an annual basis; a patient taking Invokana, Invokamet or Invokamet XR for one year has a 6 to 7 in 1,000 chance of requiring a leg or foot amputation.
Toe Amputations Most Common
Beyond basic risk estimates, the FDA has provided a few details on which sort of amputations appear to be most common. Current medical research suggests that Invokana leads most frequently to toe amputations and amputations involving the middle of the foot. Leg amputations were also observed, both below and above the knee.
Link Is Causal, FDA Says
Notably, the federal agency did not attempt to dodge the implications of these statistics. While many FDA-issued warnings are hesitant to draw a causal association, choosing instead to “suggest” that a drug “may” cause a side effect, the FDA in this instance was remarkably definitive.
In no uncertain terms, the agency wrote, “canagliflozin […] causes an increased risk of leg and foot amputations.” As a result, federal health watchdogs took immediate action, instructing Janssen Pharmaceuticals to revise the products’ warning labels.
Black Box Warning
Invokana’s label now features a prominent black boxed warning, representing the FDA’s most-severe form of warning:
“In patients with Type 2 diabetes who have established cardiovascular disease (CVD) or at risk for CVD, Invokana has been associated with lower limb amputations, most frequently of the toe and midfoot; some also involved the leg. Before initiating, consider factors that may increase the risk of amputation. Monitor patients receiving Invokana for infections or ulcers of the lower limbs, and discontinue if these occur.”
Needless to say, this warning has come too late for many type 2 diabetes patients who have already been forced to undergo an amputation procedure.
Pre-Amputation Risk Factors & Symptoms
The risk is highest in patients who have previously been diagnosed with cardiovascular disease, along with those at risk of developing cardiovascular disease. Patients who are currently taking a medication based on canagliflozin should watch for potential signs of danger:
- new pain or tenderness
- infections in the lower limbs
- ulcers or sores in the lower limbs
People with diabetes already live at an increased risk for infections and ulcers in the lower limbs, both leading risk factors for amputation.
New patients who have not yet started taking the medication should be screened for potential risk factors:
- history of prior amputation
- peripheral vascular disease
- neuropathy
- diabetic foot ulcers
As the experts at Medscape report, foot ulcers are currently the most common reason for diabetes-related hospitalization. In the United States alone, around 5% of diabetics develop foot ulcers every year; around 1% of these patients require amputation. The danger is only increased in patients with a pre-existing cardiovascular problem. Doctors have been instructed to “discontinue” use of the medication if infections or ulcers begin to develop.
Mounting Evidence Of Invokana Side Effects
Invokana is a new type 2 diabetes drug, lowering blood sugar levels by increasing the rate at which sugars are expelled from the body by the kidneys. Two other drugs, Farxiga and Jardiance, work similarly. All three medications are referred to as “SGLT-2 inhibitors.”
Due to their novel biological mechanism, SGLT-2 inhibitors were initially hailed as a potential “revolution” in the treatment of type 2 diabetes. Invokana was approved only four years ago, on March 29, 2013, but the drug’s apparent risks have become more and more pronounced.
Alongside foot and leg amputations, the FDA has warned patients about an increased risk of diabetic ketoacidosis, a form of blood acid poisoning, and bone fractures. Side effect reports have also linked the drugs to severe kidney-related disorders, including kidney failure.
Do Farxiga, Jardiance Boost Amputations?
These risks are not exclusive to Invokana; all SGLT-2 inhibitors, including Farxiga and Jardiance, appear to increase the likelihood of ketoacidosis and broken bones. Invokana’s link to leg and foot amputations, on the other hand, has not yet been attributed to other SGLT-2 inhibitors.
On this count, European regulatory bodies have advised caution. In a February report from the European Medicines Agency, the Euro zone’s top health agency recommended that warnings for leg and foot amputations should be included in the labeling for all three major SGLT2-inhibitors: Invokana, Farxiga and Jardiance. The US Food & Drug Administration has not yet made a similar recommendation, but forthcoming study results on Invokana’s competitors could change that in the future.
Learn More Today
Hundreds of patients may be able to pursue financial compensation by filing an Invokana amputation lawsuit. If you or a loved one were forced to undergo an amputation procedure after taking Invokana, Invokamet or Invokamet XR, our experienced attorneys are here to help. Contact the lawyers at Monheit Law to learn more about case eligibility and your own legal options. We offer a free consultation and offer our services on a contingency-fee basis, so you pay nothing until we secure compensation in your case. Just call us today or fill out our online form to find more information.

