Cook Medical has issued an urgent recall for medical devices used to treat severe arterial injuries:
- Zenith Alpha Thoracic Endovascular Graft
- Blunt thoracic aortic injury (BTAI)
- High risk of blood clot formation
Were you or a loved one implanted with one of Cook's Zenith grafts after sustaining a BTAI? Legal action may be possible. Call today for a free consultation.
We've helped hundreds of medical device victims pursue justice.
Monheit Law stepped in when I needed real help. I couldn't be happier with the results.
Reviewed by Sharon P. on
.
Feel free to share our Zenith recall infographic on your own site. Just copy and paste the code below:
FREE CONSULTATION
Indiana-based medical device company Cook Medical has recalled its Zenith Alpha Thoracic Endovascular Graft. Issued on March 22, 2017 and updated four months later, the urgent recall is an attempt to prevent the implant from being used in patients who have sustained blunt thoracic aortic injury, a rare but often-fatal injury sustained in motor vehicle accidents.
Cook Recalls Arterial Graft Over Blood Clot Risk
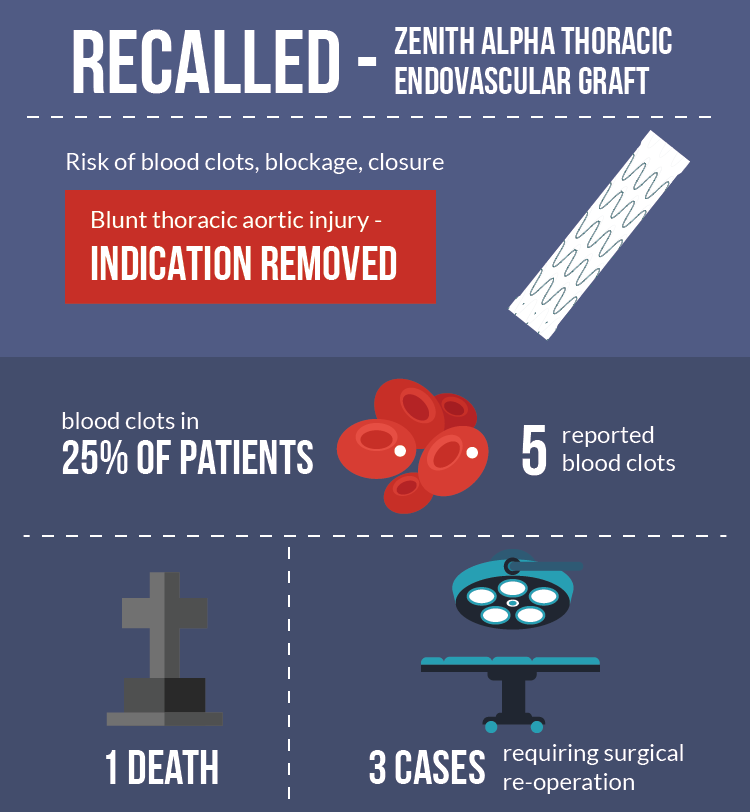
The US Food & Drug Administration has categorized the Zenith market withdrawal as a Class 1 recall, representing the most serious form of recall. Use of the Zenith Alpha Thoracic Endovascular Graft under current labeling instructions can result in serious injuries or death, the federal agency says, instructing all medical professionals to stop using the device immediately.
Around 4,500 devices will be returned to Cook and receive new labeling, after patient injury reports revealed that blood clots could form inside the graft, leading to potentially-fatal complications.
A thrombus can block the flow of blood, preventing oxygen and vital nutrients from being transported to body organs. Cook Medical has received at least 5 reports of blood clots so far, with one case leading to a patient's death. Three additional cases required surgical intervention.
The product's significant risks have been substantiated by medical research. A clinical trial conducted by Cook Medical found that, at follow-up, nearly 25% of patients implanted with a Zenith Alpha thoracic graft had developed blood clots in the device. The risks appeared to be highest, Cook says, for patients who received smaller diameter grafts and those in whom the implant's diameter was larger than the artery itself. Previous studies have found similarly troubling rates of thrombus, the company reports, with between 15% and 40% of participants affected by a clot.
Thrombus Formation, Blockage & Closure
In a warning letter sent to physicians on March 22, Cook Medical said the Zenith Alpha Thoracic Endovascular Graft, when used to treat blunt thoracic aortic injury, could promote the formation of thrombus, or blood clots, inside the device. The company went on to note a number of reports in which the implant had become blocked or occluded (closed) after implantation, a fact that has led some critics to suggest that Cook's Zenith graft may harbor a dangerous design defect.
Any of these adverse events, Cook warned, could cause severe complications, including death. An updated statement from the company came on June 22, 2017, when Cook Medical informed doctors that BTAI had been formally removed from the Zenith graft's list of acceptable indications.
Warning Label Revisions
The product's labeling has been revised to include a new warning about blood clot formation:
"Risk of in-graft thrombus has been observed when the Zenith Alpha Thoracic Endovascular Graft has been used to treat BTAI."
The manufacturer also decided to take hundreds of implants off the market completely, judging that grafts with a diameter between 18 and 22 millimeters, would likely only be used to treat BTAI, the indication that has now been prohibited. These specific sizes are no longer available for sale. A full list of affected products, including product numbers, is available here.
Cook Medical has also reminded physicians that the Zenith Alpha thoracic graft is only appropriate for certain patients. Unique anatomical considerations, including the natural diameter and curvature of a patient's aorta must be taken into account before selecting the appropriate implant.
Instructions For Current Patients
For patients who have already received Zenith Alpha grafts for blunt thoracic aortic injuries, Cook has not revised its follow-up instructions. Doctors should continue to monitor these patients as usual, following the implant's current Instructions For Use carefully.
What Is The Zenith Graft?
The Zenith Alpha Thoracic Endovascular Graft is designed to treat patients who have developed aneurysms or ulcers in the descending thoracic aorta:
- aneurysm - a swelling of the artery due to weak arterial wall muscles
- ulcer - an open wound caused by inadequate blood supply
The descending aorta is a portion of the body's largest artery, which transports blood from the heart through the abdomen. The artery's thoracic section runs downward through the chest.
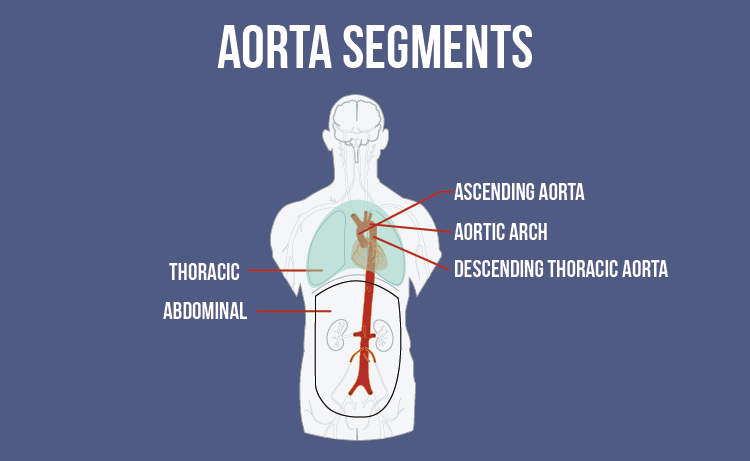
Cook Medical's Endovascular Graft is a tube of polyester fabric, structured by metal stents, that can be inserted into the artery and expanded, allowing blood to flow safely past swollen arterial walls or sites of traumatic injury.
The device was created to provide doctors with a minimally-invasive alternative to open surgical procedures, and soon came to prominence as a treatment for blunt thoracic aortic injury.
Blunt Thoracic Aortic Injury
Blunt thoracic aortic injuries (BTAI) are a rare consequence of motor vehicle accidents, according to researchers in the New England Journal of Medicine, occurring in less that 1% of car crashes. Despite its rarity, blunt aortic trauma accounts for an estimated 16% of vehicle accident-related deaths, the second-leading cause of fatality from car crashes after head injuries.
Normally caused by a sudden reduction in speed (deceleration), like that experienced in a head-on collision, the injury usually occurs when the descending aorta, a portion of artery attached to the chest wall, is violently torn from away from the heart. This separation of the aorta from the heart is often referred to as "transection," and the fatal consequences of such an injury should be obvious. An estimated 80% to 90% of all BTAI prove deadly.

